FHL1 in skeletal muscle homeostasis and pathology
Four-and-a-half LIM domain 1 (FHL1), is a member of the gene family encoding LIM domain containing proteins. A LIM domain is mainly constituted of two cysteine-rich zinc-finger motifs, which coordinately bind zinc atoms to mediate protein-protein interactions. Distinct splice variants of FHL1 have been reported, namely FHL1A, FHL1B and FHL1C, which are composed of a 1/2 LIM followed by 4, 3 and 2 full LIM domains, respectively. Alternative splicing leads to the presence of nuclear-addressing and/or RPB-J binding domains in the C-terminal part of FHL1B and FHL1C. Based on its expression pattern, which is highly enriched in striated muscles, FHL1 has been suggested to play an important role in skeletal muscle growth and remodeling. Several studies have already demonstrated a correlation between muscle growth and the levels of FHL1, and have also suggested that different muscle types, bearing different proportions of fiber types can respond differently to variations of FHL1 protein expression. However, the most significant evidence that FHL1 plays an important role in muscle development and disease comes from recent human genetic studies linking FHL1 missense mutations to congenital myopathies previously recognized by particular structural features, including Reducing body myopathy (RBM) and Emery-Dreifuss Muscular Dystrophy (EDMD). In these myopathies, different sporadic or inherited missense mutations or deletions, disrupting one of the zinc-finger structures of the LIM domain of FHL1, lead to qualitative and quantitative changes in FHL1 expression, resulting in mild-to-severe muscle wasting and dystrophy.
The overall goal of our study is to understand the role that FHL1 plays in skeletal muscle homeostasis and why different mutations in FHL1 result in different forms and severity of diseases. We will achieve our goal by studying an FHL1 knockout mouse line that we generated in our laboratory and by creating additional mouse lines in which point mutations will be introduced through gene targeting in order to mimic mutations identified in RBM and EDMD, followed by comprehensive molecular, biochemical, histological, and physiological analyses of their skeletal phenotypes.
Analysis of FHL1 functions - new manuscript
August 2013. Recent studies provided evidence that inherited missense mutations in four-and-a-half LIM domain protein 1 (FHL1) are associated with rare myopathies, including reducing body myopathy and Emery-Dreifuss muscular dystrophy. Our study, recently published in the Journal Human Molecular Genetics evaluates loss of FHL1 functions on skeletal muscle homeostasis.
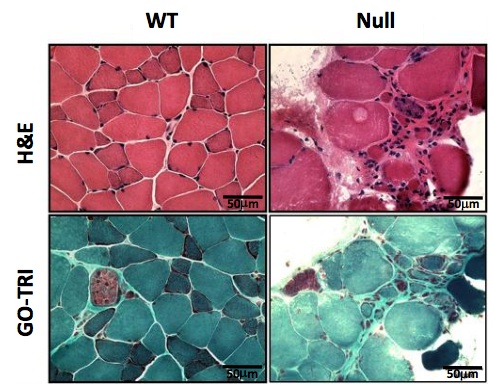
Using a wide array of techniques, we show that skeletal muscles from FHL1 null mice develop an age-dependent myopathy associated with myofibrillar
and intermyofibrillar (mitochondrial and sarcoplasmic reticulum) disorganization, impaired muscle
oxidative capacity and increased autophagic activity. Decreased survival rates in FHL1-null mice were associated with age-dependent impairment of muscle contractile
function and a significantly lower exercise capacity. These data highlight that FHL1A is necessary for proper muscle
fiber differentiation and maturation in vitro.
Click here for the full article > (Subscription may be required).
Section 'Sub' Navigation:
Page 'Breadcrumb' Navigation:


